How Does Pool Salt Work? How Much Salt To Add To Pool?
2021-06-04(30263)
Adding salt to water is a commonly used method for disinfection of swimming pools. Furthermore, salt water pool treatment stands out with its numerous advantages compared to other disinfection methods.
In this article, we will explain the specifics of pool disinfection with salt, and the ideal salt level for the pool.
Salt as A Swimming Pool Disinfectant
Salt treatment does not require routine maintenance. The device called chlorinator works continuously to transform the salt (NaCl) into chlorine and then converts the NaCl to its original state. This cycle repeats constantly. As a result, the chlorine is continuously renewed.

The chlorine generated as a result of this process is not as harmful as the chlorination method. It does not damage the skin and does not create a strong odor in water.
Besides, salt water pool treatment is the healthiest and most cost-effective method of disinfection continuously. It reduces pool maintenance costs by 80% and eliminates the use of disinfectant chemical products entirely.
Pool Disinfection with Electrolysis
The most widely used water disinfection method in the world is electrolyzing with salt for pool. It is also the most recommended method both economically and environmentally. A small amount of salt in the water serves as a natural antiseptic, preventing the growth of bacteria and algae while causing no damage to swimmers' skin.
Chlorine has a much better hygienic influence than conventional systems. At a certain point in the water disinfection cycle, chlorine is continuously created, and its concentration reaches extremely high levels. This high chlorine concentration removes contaminants that cannot be eliminated at the pool's current chlorine level.
Furthermore, as water passes through the electrode unit, it is exposed to a strong electrical field, which causes contaminants to oxidize and destroyed. In other words, with this method, two times stronger hygiene and sterilization can be achieved. However, it is essential to be careful about how much salt to add to pool to create an ideal environment for the process to succeed.
What is Electrolysis?
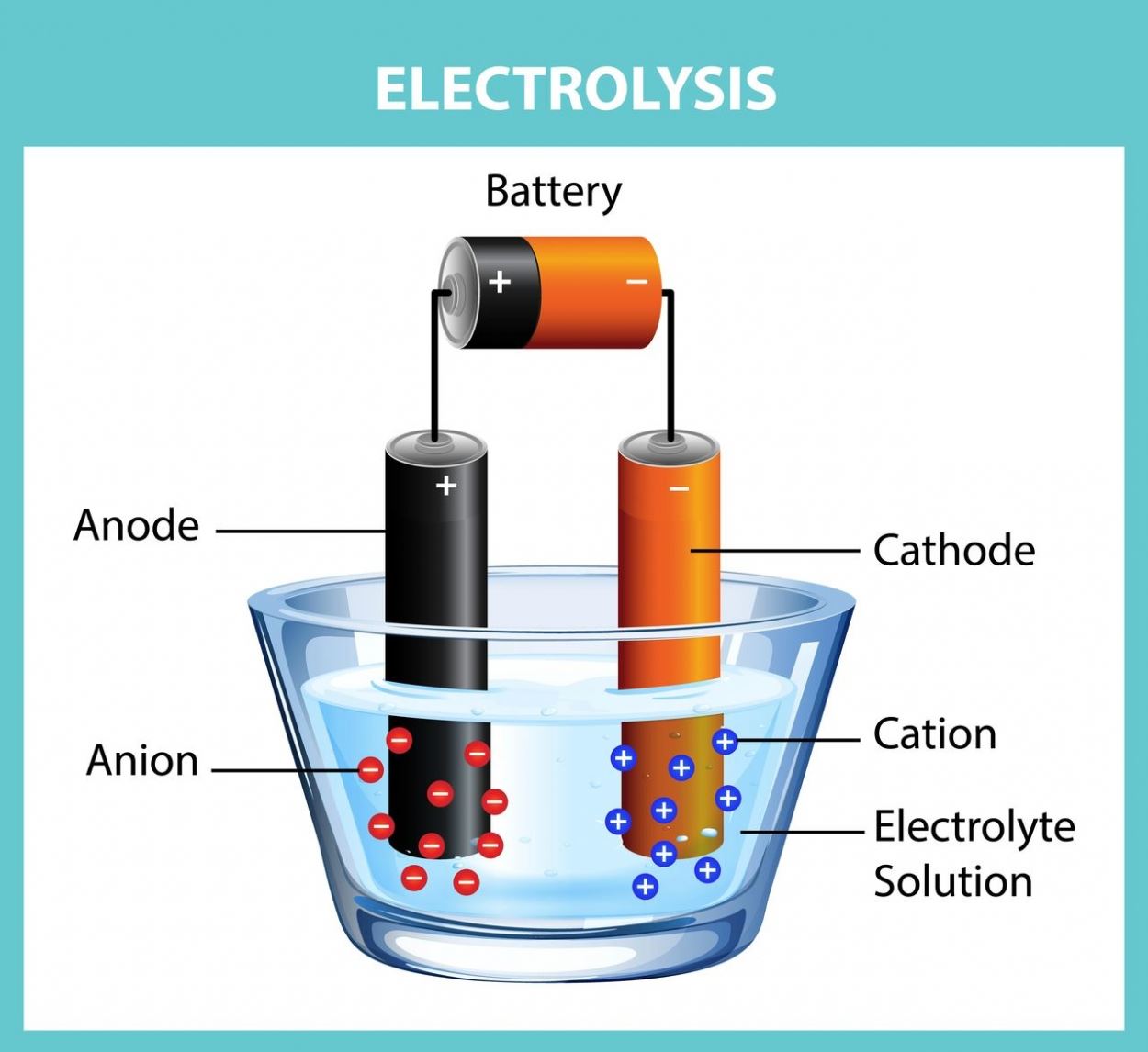
Electrolysis is the decomposition of chemical compounds dissolved in a liquid using an electric current delivered by external electrodes. Electrolysis may be performed by a variety of substances. Salts (as in pool salt), acids, and bases are the most well-known examples.
For electrolysis, an external power source, electrodes, and electrolytes are required. The term "electrolyte" refers to the environment in which free ions exist. The electrode is a term used to describe conductive metals that have been soaked in electrolytes.
The electrolysis process occurs inside the electrolysis container or tank, which is also called a “cell”. Inside the container are two electrodes immersed in a compound that dissolves and is divided into plus (+) and minus (-) charged ions. These electrodes are adjusted so that they do not touch each other, usually with 5-20 centimeters (approx. 4-8 inches) between them.
The electrodes are connected to a direct current source, and the voltage between the electrodes pushes the ions towards the oppositely charged electrode. After the procedure, (+) loaded ions flow towards the cathode, while (-) loaded ions flow towards the anode. At the opposite pole, atoms or molecules that balance their load precipitate and react with other molecules in the electrolyte.
Among chemical compounds, there are two types of bonds: covalent and ionic. While it is not possible to separate the compounds with covalent bonds, it is possible to separate the compounds with ionic bonds via electricity.
How Much Salt to Add to Pool?

For the salt electrolysis for the pool disinfection process to work, a very diluted saline solution must first be provided. To create this environment, salt between 4 - 6 kilograms per cubic meter (approx. 9 - 13 lbs) should be dissolved in pool water.
The resulting solution has a salinity that is 9 times lower than seawater. This value creates a suitable environment for the chlorinator.
For complete disinfection, salt should be added to water to achieve a salinity rate of 0.4%. To obtain the ideal salt level for the pool, if the pool is devoid of salt, 16 kg (approx. 35 lbs) of salt should be applied for every 4000 liters (approx. 1000 gallons).
Table Salt vs. Pool Salt
One of the most frequently asked questions about pool salt is whether pool salt can be used in foods or whether it is possible to use table salt for pool disinfection. This curiosity stems from the thought that pool salt contains chemicals.
In fact, the salt used to disinfect pools must be 99.8% pure. Pool salt does not contain any anti-clumping agents and it does not go through the same iodization process as table salt. Although many people may believe that table salt is the purest of the two, pool salt is purer.




